What is an electrolyzer?
An electrolyzer is a system that performs electrolysis, which is the process of using electricity to split water molecules (H2O) into hydrogen (H2) and oxygen (O2). Zero-carbon hydrogen can be produced if the electrolyzer is powered by wind, solar, or nuclear sources of electricity. For this reason, electrolyzers are expected to play a significant role in decarbonizing energy, refining, transportation, chemical production, and steel manufacturing.

What are the different types of electrolyzers?
There are several types of electrolyzers, but they all split water molecules to produce hydrogen and oxygen. Electrolyzers are differentiated by their materials of construction. Low-temperature electrolyzers use a polymer electrolyte membrane (PEM) or a more porous membrane filled with alkali solution (Alkaline).
Both PEM and Alkaline electrolyzers operate at a little higher than room temperature and have electrical efficiencies between 65 and 75 percent (compared to the higher heating value of hydrogen). This electrical efficiency refers to the electrical energy required to produce hydrogen.
Solid oxide electrolyzers (SOECs), however, have a ceramic electrolyte and run at higher temperatures, which allows them to operate at an impressive 90 percent efficiency. This higher efficiency requires less electricity consumption, significantly reducing the cost of producing hydrogen.
How does a solid oxide electrolyzer work?

A solid oxide electrolyzer cell consists of the cathode (the negative electrode), the anode (the positive electrode), and the electrolyte. When a voltage is applied between the cathode and anode, an electrical current is produced which flows through the cell. As can be seen in the above diagram, water in the form of steam enters the cathode side of the cell. The electrical current drives an electrochemical reaction which splits the water into hydrogen and oxygen.
The electrolyte of a solid oxide cell has a unique property that can transport oxygen ions but is impermeable to hydrogen. As a result, hydrogen is concentrated on the cathode side and exits the cell as pure hydrogen plus some residual steam. The cells are configured into stacks, and multiple stacks are used in the electrolysis system. One key aspect of solid oxide electrolysis cells is that they do not require noble metal catalysts such as platinum or iridium, avoiding higher costs and critical material supply issues.
An electrolysis system consists of the electrolysis stacks plus support equipment, commonly referred to as a “balance of plant.” FuelCell Energy’s solid oxide system is a packaged platform that includes equipment for making steam from input water, and equipment for cooling and drying the product hydrogen. This equipment is packaged with the stack in one skid, and a second skid contains the electrical equipment (for making DC power from AC input) and control equipment.
Solid oxide electrolyzer efficiency advantage
Electricity consumption is the largest factor in the cost of hydrogen produced by electrolysis. Higher electrolyzer efficiency reduces electricity consumption and lowers the cost of hydrogen produced. FuelCell Energy’s SOEC system operates at approximately 90 percent efficiency using electricity and its own recovered heat to boil electrolysis feedwater. It is important to note that this 90 percent efficiency is the full system efficiency. It includes the parasitic losses for balance of plant mechanical and electrical equipment on top of any stack inefficiencies (which are very low).
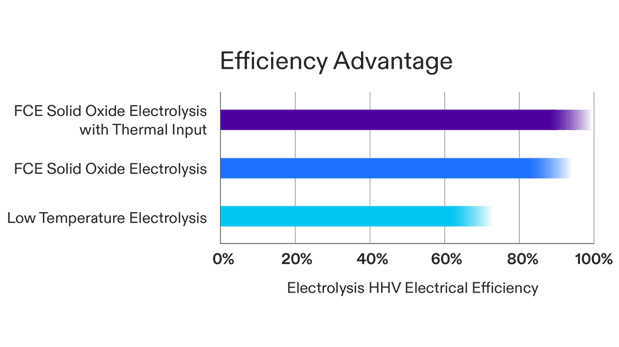
External heat is not required for our electrolyzer to operate, however, heat from external sources can be used in our SOEC to boil feedwater and reduce electricity consumption. The mechanical skid includes a provision for the optional supply of waste heat, which would increase the efficiency to almost 100 percent, about 35 percent more efficient than low-temperature electrolyzers. Waste heat from a nuclear power plant or industrial sources can be used to provide this efficiency increase. If external heat is no longer available (such as during maintenance periods of the heat source), the SOEC system can continue to operate with electricity as the sole energy input.

